Documents
Ozone White paper
The white paper below was written by Dr. Ian Van Trump, a microbiologist who provides both scientific and regulatory support for the research, development, state/federal registration, and ongoing regulatory compliance of various products regulated by the U. S. Environmental Protection Agency (EPA) and other agencies. Dr. Van Trump also has experience with the premarket clearance of medical devices such as high-level disinfectants, wound cleansers, and other devices regulated by the U.S. Food and Drug Administration (FDA) which incorporate antimicrobial technologies. A shortened version of the full paper is also available.
Lab Reports
The CerroZone mobile device has successfully passed extensive testing at a number of accredited third-party laboratories and industry certification organizations. Reports shown here may have certain proprietary information redacted, but no test procedures or results have been edited. Full reports can be made available after execution of a binding non-disclosure agreement. Contact sales@cerrozone.site if interested.
Brochures
Treatment Room White Paper
This case study uses computational fluid dynamics to investigates the effect of airflow generated by the CerroZone mobile air purifier on laminar air supply in operating rooms.
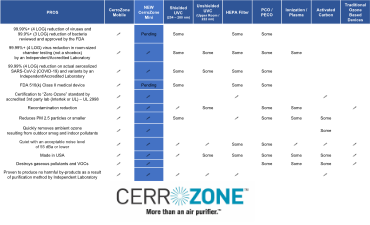
Mini Unit Comparison Matrix
2023 ASHRAE Winter Conference Seminar: Infection Control in Commercial Spaces

NIH Article on Airborne PCB Exposure
Certain information set forth in this website and in these documents contains “forward-looking statements”. Although these forward-looking statements are based upon what management of the Company believes are reasonable assumptions, there can be no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements.












